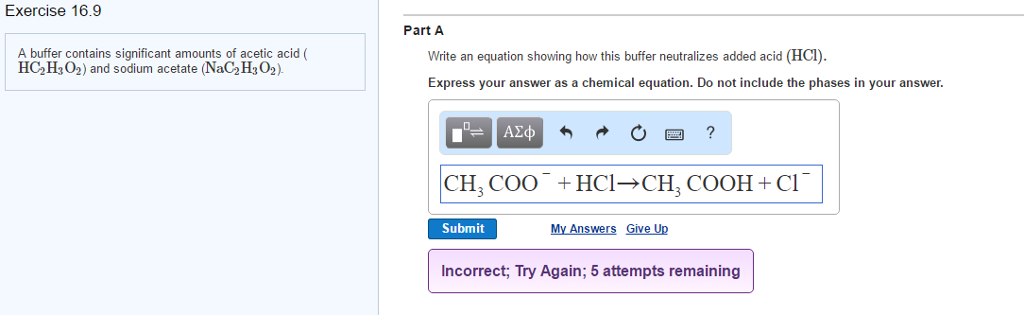
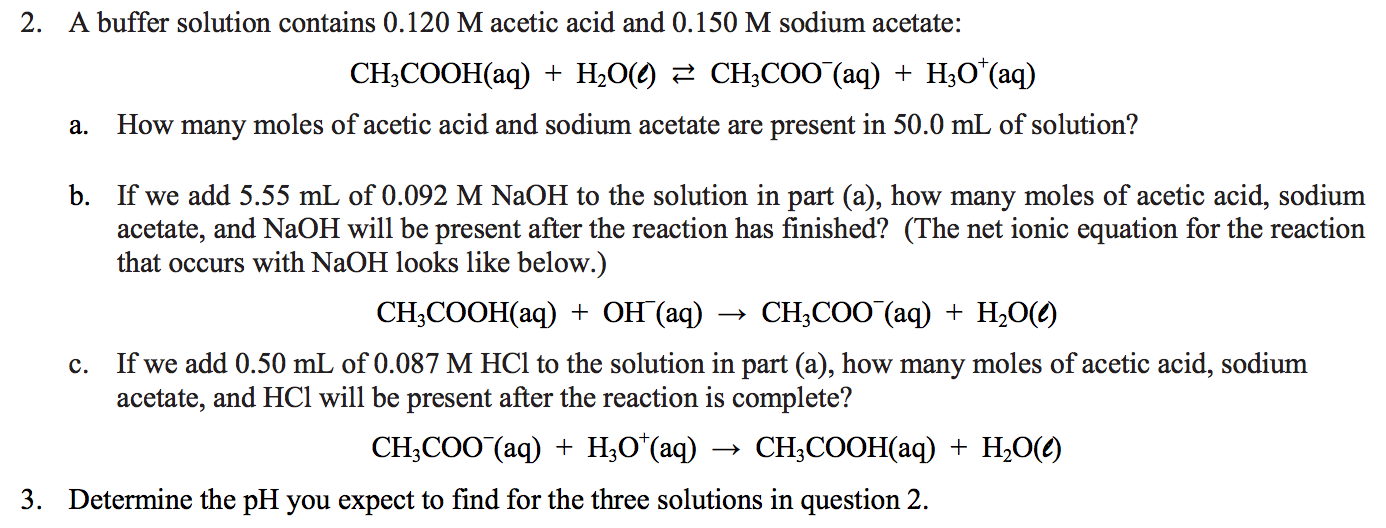
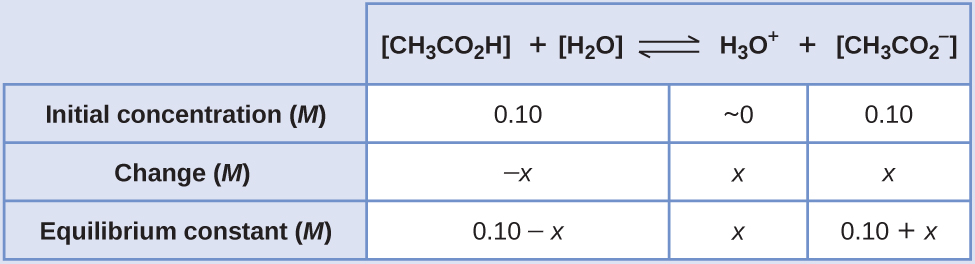
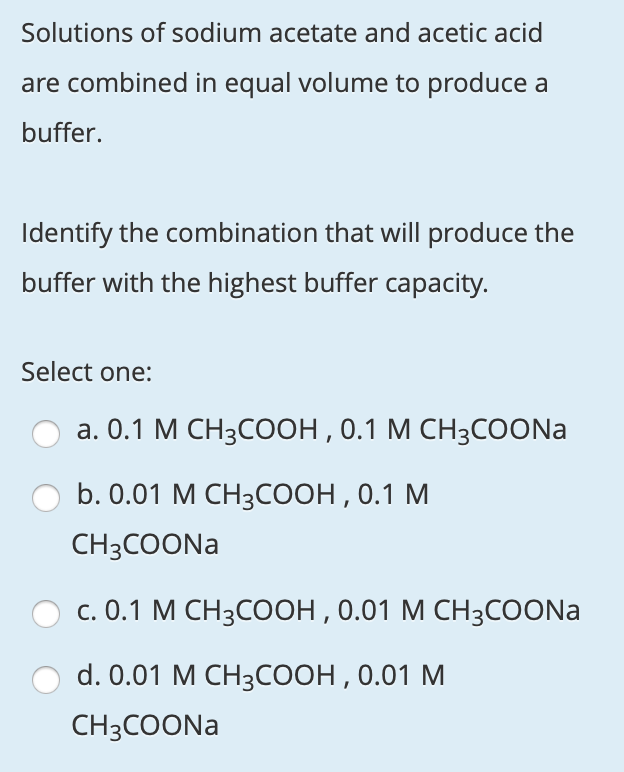
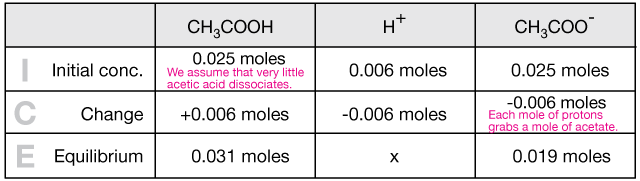
OneClass: A buffer contains significant amounts of acetic acid and sodium acetate. Write an equation ...

Titration of CH3COONa with HCl and pKa determination from half equivalence point - Chemistry Stack Exchange
How to prepare 1oo ml of 0.200 M acetate buffer at pH 5.00 starting with pure liquid acetic acid and solutions containing 3M HCl and 3M NaOH - Quora
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://i.ytimg.com/vi/t9B5VgPOTG4/maxresdefault.jpg)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]

A buffer solution is prepared by mixing `10ml` of `1.0 M` acetic acid & `20 ml` of `0.5 M` - YouTube

BUFFERS Mixture of an acid and its conjugate base. Buffer solution resists change in pH when acids or bases are added or when dilution occurs. Mix: A. - ppt download

E-Lifes: Acetate buffer preparation and calculation. (Weak acid + salt of weak acid , Weak acid + salt of weak acid, Weak acid + strong acid)
Compare 1 L of acetate buffer solution (0.50 mol of acetic acid and 0.50 mol sodium acetate) to 1 L of HCl solution Similarities